The important components of milk are the milk-sugar lactose and the proteins casein, lactalbumin, and lactoglobulin. These may be incorporated into a standard agar medium along with litmus (redox pH indicator) in order to observe microorganisms that may possess the ability to metabolize lactose and the milk-proteins.
Using the litmus-milk medium, it is possible to test for: lactose fermentation; gas production; litmus reduction; curd formation; proteolysis; and alkaline reaction.
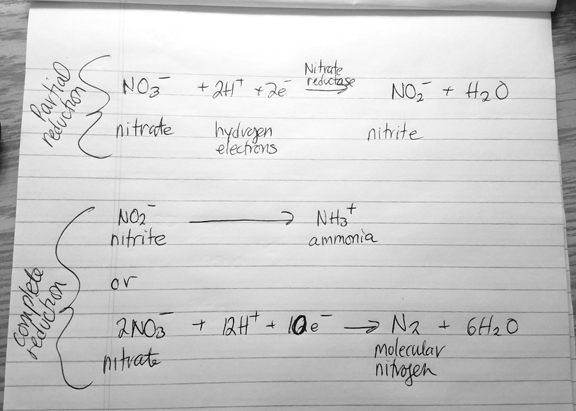
Lactose fermentation. Lactose may be used as an alternative carbon source by organisms capable of producing beta-galactosidase. Beta-galactosidase helps to break down lactose into glucose and galactose which can then enter the glycolytic (Embden-Meyerhof) pathway. The end product, lactic acid, may then be detected by the pH indicator: litmus is purple when there is no change (negative result); litmus turns pink when the medium is acidic up to pH 4.
Gas formation. Gases likely to be produced are carbon dioxide and hydrogen gas. Tiny fissures/breaks/bubbles in the agar medium may indicate such gas formation.
Litmus reduction. Lactose oxidation produces carbon dioxide and hydrogen gas. Litmus acts as an electron acceptor as indicated by a white or milk-ish color.
Curd formation. It’s possible that curds/clots may form as a byproduct. Curds are acidic or rennet. Acid curds: are precipitates (calcium caseinate) of lactic acid or other organic acid; hard and usually sticks firmly to sides of a test tube (especially if tube is inverted). Rennet curds: are precipitates that from when rennin (enzyme) acts on casein forming paracasein; paracasein plus calcium ions converts to calcium paracaseinate, an insoluble semisolid curd/clot which will flow slowly when test tube is inverted.
Proteolysis (peptonization). Some organisms cannot metabolize lactose for energy, but they may be able to metabolize milk-proteins for energy instead. As milk-proteins (usually casein) are broken down into their component amino acids, the byproduct ammonia causes the pH of the medium to turn alkaline. Litmus will turn deep purple at the upper part of the tube, and the medium may turn translucent brown or become whey-like.
Alkaline reaction. When casein is only partially metabolized into short polypeptide chains, the medium turns alkaline (alkaline reaction). The medium’s color remains unchanged or it may change to a deep blue.
To summarize…
Litmus milk results:
A. Pink—lactose fermentation (acid)
- Pink band up top + white color medium below—acid followed by reduction.
- Pink band up top + white color medium below + solids—acid, reduction, and curd.
- Pink band up top + white color medium below + solids + fissues/cracks/bubbling—acid, reduction, curd, and gas.
B. White + purple band up top—litmus reduction.
C. Deep puple band up top + translucent whey-looking brown medium—proteolysis.
D. Medium unchanged or is a deep blue color—alkaline reaction.
Clinical significance. Litmus-milk is a differential medium for Enterobacteriacaeae and other gram-negative bacilli.
Reference
Cappuccino, J. G., & Welsh, C. (2018). Microbiology: A laboratory manual.