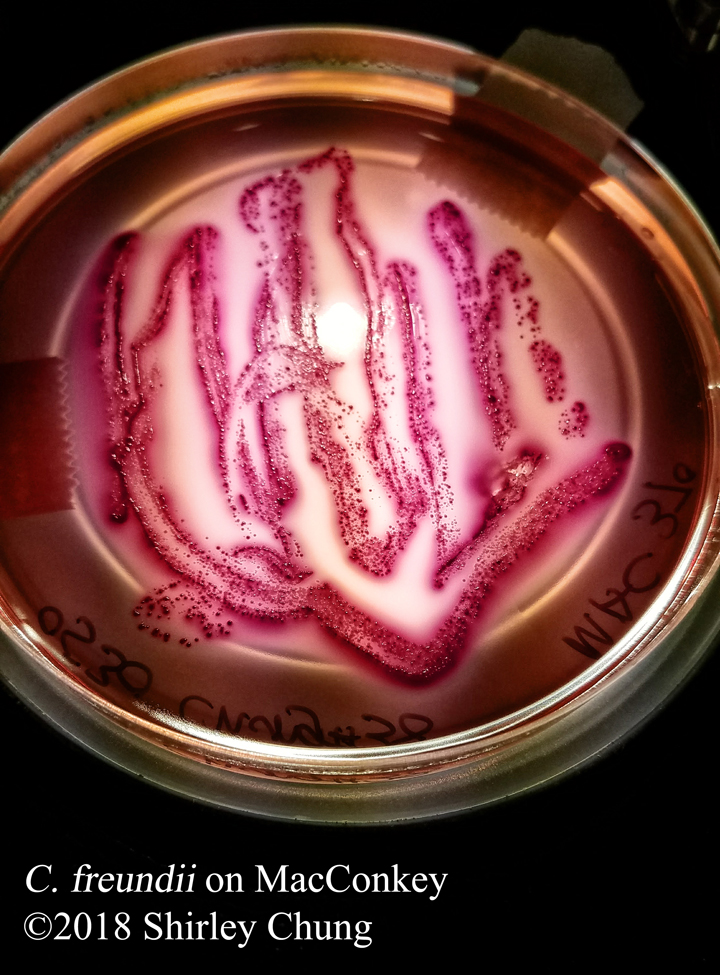
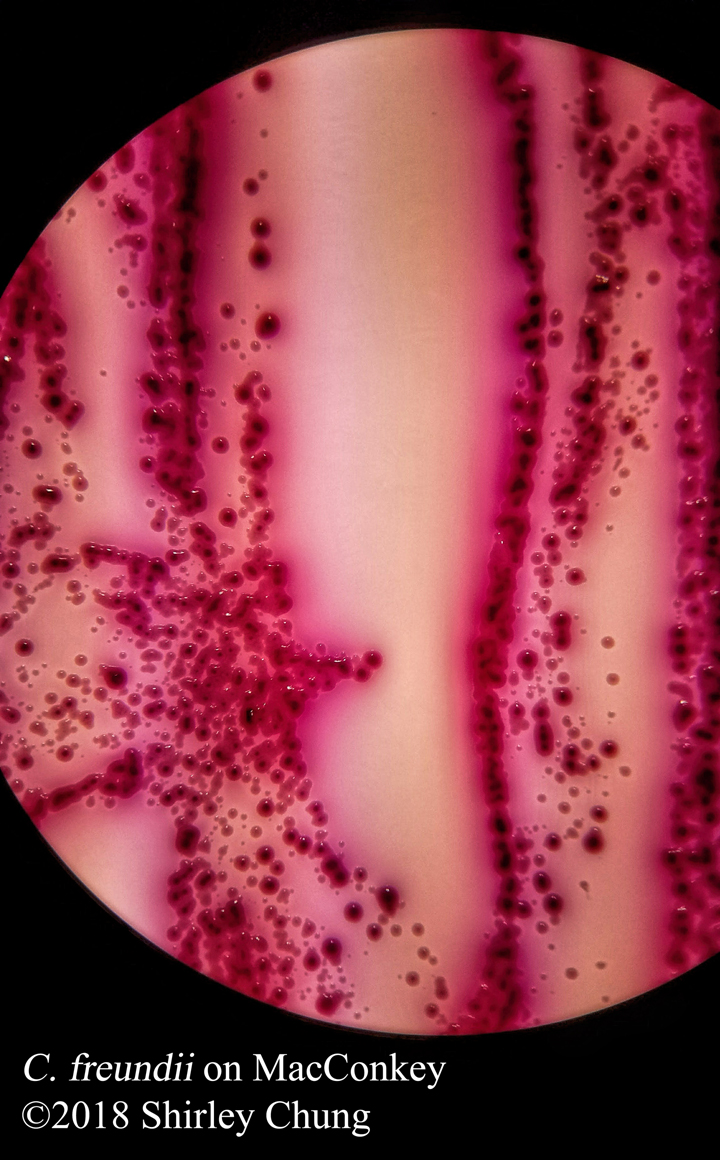
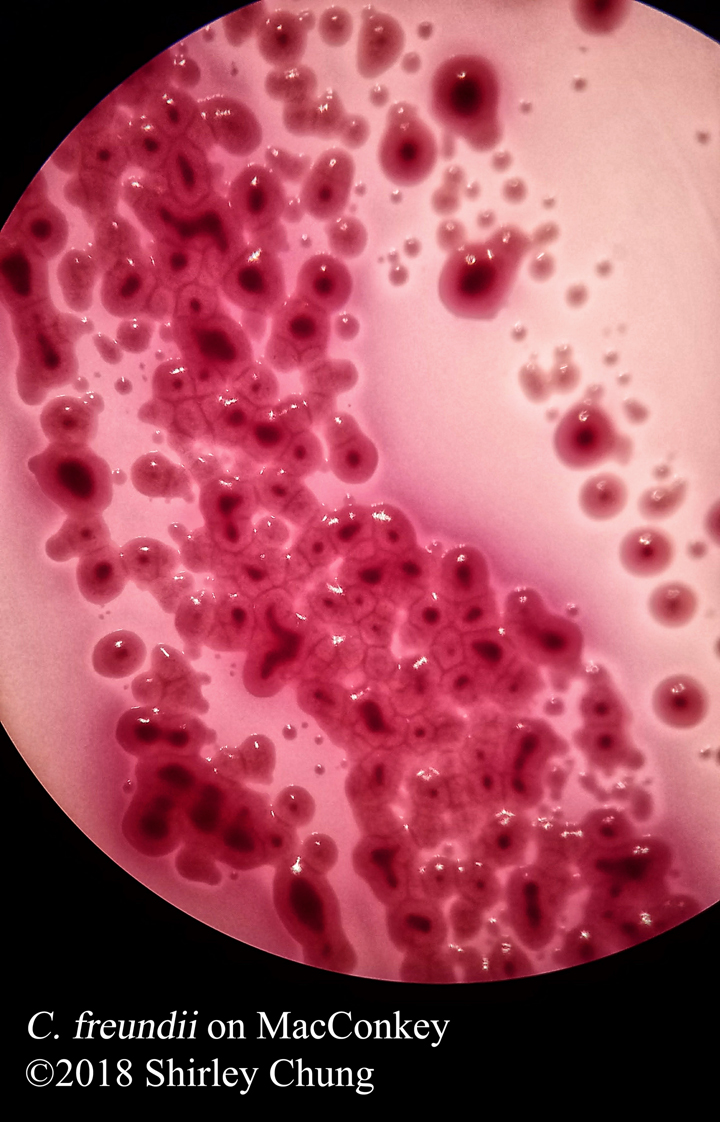
Negative stains: uses an acidic stain like India ink or nigrosin. The anionic chromogen properties of the stain will not penetrate the cell/organism thereby staining the “background” (negative space around the cell/organism) instead. Heat fixing is not required so beware that the organisms/bacteria are NOT killed. Aseptic technique and proper disposal is important.
Clinical significance. Helpful in detecting spirochetes (difficult to stain), presence of a capsule or spore. Helps identify infectious agents such as fungi from bird droppings.
Summary of procedure.
- Place a drop of India ink or nigrosin on one end of a clean slide.
- Use a loopful of inoculated broth and mix it well with the drop of stain on the slide.
- Place the short edge of another slide at a 45 degree slant against the spot of stain. Drag this second slide across the original slide (smearing the drop across).
Reference
Cappuccino, J. G., & Welsh, C. (2018). Microbiology: A laboratory manual.