Dilutions serve to reduce the concentration of something. In microbiology, dilutions reduce the concentration of an organism per unit volume. For example, one may need to reduce the microbial concentration in order to be “countable” or run a test/diagnostic (e.g. efficacy of an antimicrobial drug).
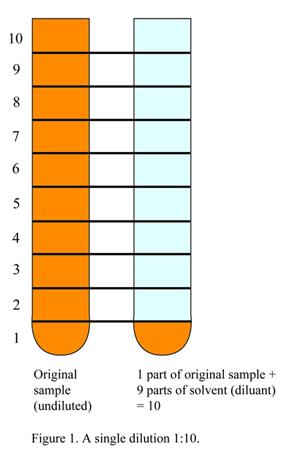
A [single] log dilution means the concentration is decreased by a factor of 10 or 10^1 (10 raised to the power of 1) or 1:10. The “1” is one unit volume of solute, and the “10” is the total unit volume (which in this case means that there are 9 unit volumes of solvent). Refer to Figure 1.
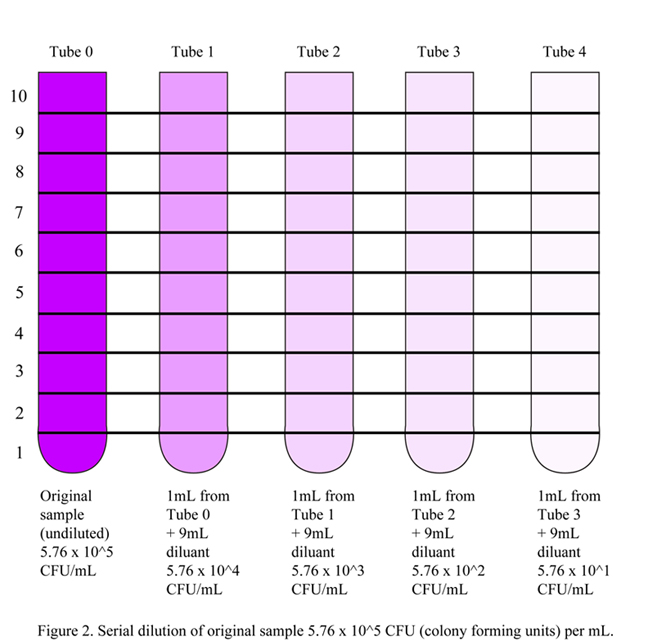
In serial dilutions, each dilution decreases the concentration of the solute by a factor of 10 by repeatedly taking 1/10th of the previous diluted solution and adding it to 9/10ths of solvent (also called diluant). Refer to Figure 2.
A great video I found is (click here) by Microbiologics.
Reference
Cappuccino, J. G., & Welsh, C. (2018). Microbiology: A laboratory manual.